7. Triple G
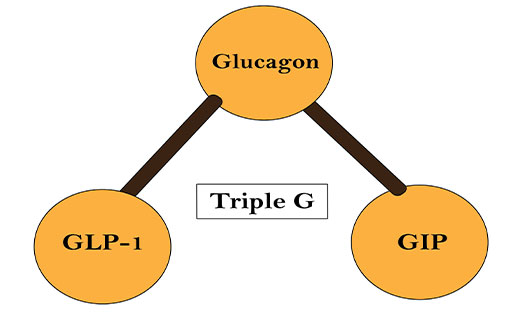
Triple G is a single peptide with agonist activity at the glucose-dependent insulinotropic polypeptide (GIP), GLP-1, and glucagon receptors. This is administered as a once-weekly injected drug and belongs to incretin class. The mechanism of this drug is designed to mimic the action of the GLP-1 hormone, which helps regulate blood sugar, slow stomach emptying and decrease appetite. Triple G, currently in development for obesity, type 2 diabetes (T2D), and non-alcoholic fatty liver disease (NAFLD), is of special interest due to addressing the requirement for better efficacy in obesity management.
Even though, the drug shares similarity with other treatments for obesity, including the gold standards, Novo Nordisk’s Wegovy (semaglutide) and Saxenda (liraglutide), Triple G stands out by offering a unique feature unlike all other GLP-1RA treatments: it employs a triple-agonist (triple-G) mechanism of action. In addition to being a GLP-1RA, it also targets gastric inhibitory polypeptide receptor (GIPR), and glucagon receptor (GCGR). The lead molecule has been the latest topic for discussion among health care professionals as it has consequently shown increased efficacy benefits in clinical trials, superior to those seen with Wegovy, which facilitates around -15% body weight loss, and Eli Lilly’s tirzepatide, which is currently in the late-stage pipeline, which employs a dual-agonist mechanism of action via GLP-1R and GIPR, and enabled -22.5% body weight loss.
The manufacturer of the drug, Eli Lilly, and the research urges that the results, if confirmed in a larger phase 3 study, could be revolutionary for people with obesity.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter




