7. Drug Update
FDA approves Bexagliflozin for type 2 diabetes
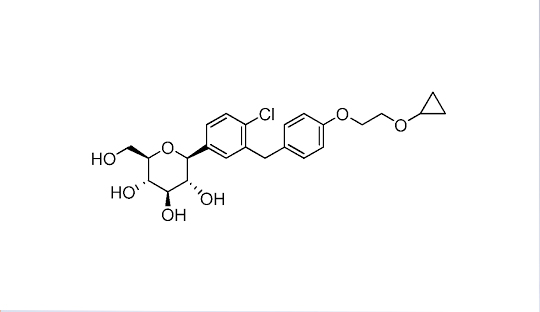
The US FDA has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes. The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min/1.73m2. The drug has proven efficacy in significant A1c reduction and fasting blood glucose at 24 weeks as monotherapy or as an add-on to metformin and other glucose-lowering drugs and combinations. It also produced considerable reductions in body weight and systolic blood pressure.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter




