
The Diabetes Control and Complications Trial/ Epidemiology of Diabetes Interventions and Complications (DCCT/ EDIC) study continues to address knowledge gaps in our understanding of type 1 diabetes and the effects of intensive therapy on its long-term complications. During the DCCT (1982–1993), a controlled clinical trial of 1,441 subjects with type 1 diabetes, and the EDIC (1994–present), an observational study of the DCCT cohort, core data collection has included medical history questionnaires, surveillance health exams, and frequent laboratory and other evaluations for microvascular and macrovascular disease. Numerous collaborations have expanded the outcome data with more detailed investigations of cardiovascular disease, cognitive function, neuropathy, genetics, and potential biological pathways involved in the development of complications.

Intensive therapy during the DCCT significantly reduced the risk of Diabetic Peripheral Neuropathy(DPN) and Cardiovascular Autonomic Neuropathy(CAN) at DCCT closeout (64% and 45%, respectively, P < 0.01). The prevalence and incidence of DPN and CAN remained significantly lower in the DCCT intensive therapy group compared with the DCCT conventional therapy group through EDIC year 13/14.

DCCT INT(Intensive Diabetes Therapy) and lower levels of HbA1c during DCCT/EDIC were associated with thinner carotid IMT, less coronary calcification, and a lower incidence of clinical cardiovascular events including myocardial infarction, stroke, and cardiac death. While there were no significant differences in cardiac structure and function be- tween the former INT and CON(conventional)groups, they were significantly associated with higher HbA1c during DCCT/EDIC.

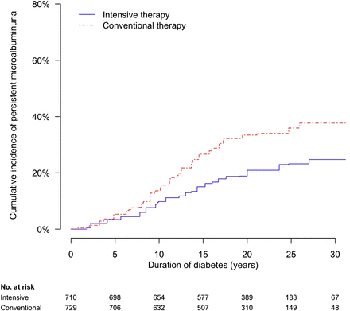
During the DCCT, INT reduced the risks of incident microalbuminuria (AER ≥ 40 mg/ 24 h) and macroalbuminuria (AER ≥ 300 mg/24 h) by 39% (95% CI 21–52%) and 54% (29–74%), respectively. During EDIC years 1–8, participants previously assigned to DCCT INT continued to experience lower rates of incident microalbuminuria and macroalbuminuria, with risk reductions of 59% (39–73%) and 84% (67–92%), respectively. Beneficial effects of INT on the development of impaired GFR (sustained estimated GFR <60 mL/min/1.73 m2) and hypertension became evident during combined DCCT/EDIC follow-up, with risk reductions of 50% (18–69%) and 20% (6–21%), respectively, compared with CON.
