4. Nasal Glucagon Successfully Treats Severe |
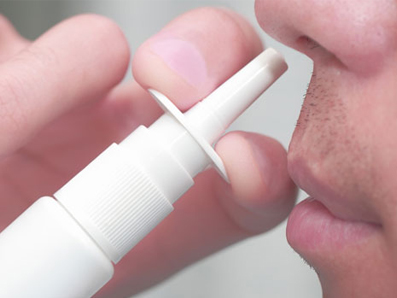
![]() A puff of dry glucagon, developed by Eli Lilly, is being tested as a potential alternative to injectable glucagon which is currently the leading treatment for severe hypoglycemia outside of a hospital setting.
A puff of dry glucagon, developed by Eli Lilly, is being tested as a potential alternative to injectable glucagon which is currently the leading treatment for severe hypoglycemia outside of a hospital setting.
![]() This phase III trial found administering one 3-mg dose inside the nose was easy for a caregiver to do in a home setting. Because injectable glucagon requires several steps, researchers from the University of Minnesota School of Medicine, Minneapolis believe simplicity is key to the product’s potential.
This phase III trial found administering one 3-mg dose inside the nose was easy for a caregiver to do in a home setting. Because injectable glucagon requires several steps, researchers from the University of Minnesota School of Medicine, Minneapolis believe simplicity is key to the product’s potential.
![]() A total of 157 episodes of hypoglycemia were experienced by 69 patients in the trial. In 151 cases, the patients returned to normal or awakened within 30 minutes of drug administration.
Lead author Dr Elizabeth R Seaquist confirmed that the 3-mg dose has also been tested in children with type 1 diabetes and could too be used by people with type 2 diabetes.
The findings were presented at the recent American Diabetes Association 2017 Scientific Sessions.
A total of 157 episodes of hypoglycemia were experienced by 69 patients in the trial. In 151 cases, the patients returned to normal or awakened within 30 minutes of drug administration.
Lead author Dr Elizabeth R Seaquist confirmed that the 3-mg dose has also been tested in children with type 1 diabetes and could too be used by people with type 2 diabetes.
The findings were presented at the recent American Diabetes Association 2017 Scientific Sessions.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter

