2. Can an advanced lancing device alleviate pain and improve HbA1c?

This study was presented at Advanced Technologies & Treatments for Diabetes, 14th International Conference, Virtual, June 2021.
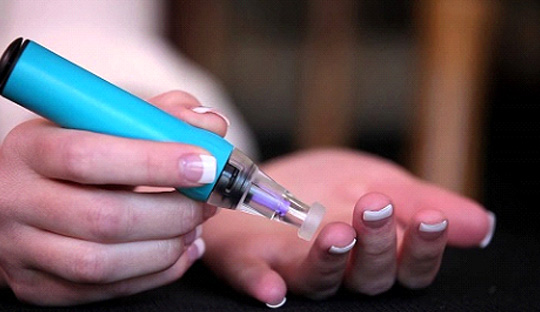
Pain has been perceived as a major impediment to self monitoring of blood glucose (SMBG). To alleviate the burden of the pricking pain, various clinical trials were initiated and are ongoing. In line with this, the research team at Jothydev’s Diabetes Research Centre assessed the benefits of using Genteel, a vacuum based lancing device in improving HbA1c and pain of pricking.
This is an ongoing, open‐label, 24‐week cross over trial where patients with diabetes were matched using propensity score and allocated to GC or CG arm (G‐ Genteel; C‐ Conventional). GC arm exclusively used Genteel for 12 weeks, and then switched to the conventional method of SMBG for additional 12 weeks, and vice versa for CG arm. A total of 110 patients were recruited with 55 in each arm. Both arms were provided with the same glucometer. CG arm used the lancet and lancing device which they were using prior to randomization and GC used BT Lancets during the first 3 months. Primary outcomes were a reduction in HbA1c and %SMBG adherence over 24‐weeks. Subjective assessment of pain in both arms was assessed.
The interim results of the ongoing trial from 22 patients (13, type 1 patients age: 25 ± 8.29, duration of diabetes 10 ± 7.12y and 9 T2DM, age: 43 ± 13.40, duration of diabetes: 11 ± 6.75y) showed a significant reduction in A1c in both arms while using Genteel(9.05 ± 0.93% at baseline to 7.76 ± 0.84% at week 12 in GC arm and 7.52 ± 1.22% at 12 to 7.21 ± 0.90% in CG arm at 24 weeks; p < 0.001*).This was reinforced by increased SMBG adherence to genteel due to alternate testing sites and contact tips.
The study demonstrates Genteel superior in terms of A1c reduction and pain of pricking.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter




