2. Real-World Performance of IDegAsp over Six Years in Asian-Indian Population
This article which evaluated the performance of IDegAsp in the Asian-Indian population is presented at the American Diabetes Association (ADA) annual meeting 2022 by the diabetes team from Jothydev’s Diabetes Research Centre. There is scarce data on the long-term safety and efficacy of IDegAsp in Asian Indians in a real-world (RW) setting. IDegAsp is a co-formulation of an ultra-long-acting insulin Degludec and a rapid acting insulin Aspart.
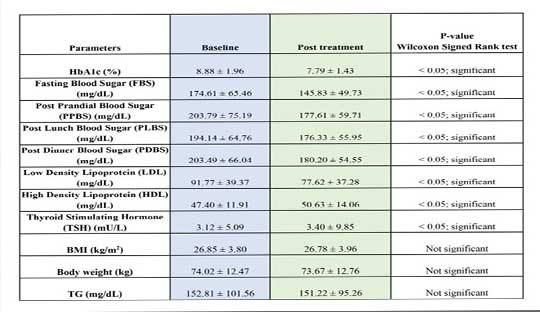
A multi-centric, retrospective observational study was conducted to evaluate the long-term efficacy and safety of IDegAsp in T2D on regular follow up for >5 years. Baseline clinical data of 535 T2D (78.7% males; avg. age= 53.41 ± 12.yrs; HbA1c= 8.88 ±1.96 %; avg. duration of diabetes: 11.34 ± 7.57yrs) , previously on treatment (90.1 %) which included various OADs±insulin regimen, or treatment-naive (9.9 %) , who were switched to or initiated on IDegAsp respectively, from December 2014 - 2021 were analyzed.
Significant improvements (P< 0.05) in clinical parameters were observed from baseline. The TDD was 15.45 ± 10.53 units/day (at 3 months) which increased (p< 0.001) to 20.03 ± 11.units/day post-treatment (67 months) . 65.2 % continued twice daily IDegAsp and 3.9 % switched from twice daily to once daily IDegAsp during treatment. 30.9% continued once daily IDegAsp. A total of non-severe hypoglycemic episodes were observed. In this RW study involving T2D from Asia, IDegAsp significantly improved A1C and a range of clinical profiles, with negligible hypoglycemia. Unlike conventional premix/basal bolus regimen, IDegAsp offers a less complex, more physiological, safe and effective solution for glycemic control.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter




