
6. Drug Update
FDA update on NDMA contamination in Metformin

Metformin is a popularly known prescription drug to control high blood sugar in
patients with type 2 diabetes. According to recent reports by international health
authority laboratories, Metformin was suspected of being contaminated with
N-nitroso-dimethylamine (NDMA), a probable human carcinogen. To examine the
level of NDMA in Metformin drug products and drug substances and to assist the
on-going investigations, a liquid chromatography-high resolution mass spectrometry
(LC-HRMS) method was developed and validated by the FDA. The investigations using
LC-HRMS summarized the findings on the limit of detection (LOD), limit of quantitation
(LOQ) and range of the method as follows:
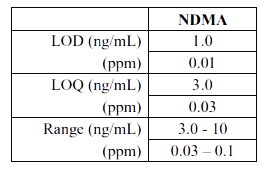
Based on these results, the FDA revealed their decision in a press release that the level of NDMA is within the acceptable range and there is no harm in continuing the usage of the drug. As per the FDA to date, no sample of Metformin that FDA has tested exceeds the acceptable daily intake for NDMA and therefore is safe. FDA advised patients not to stop using Metformin without the consent of their health care professionals as it could be dangerous for patients with any serious condition to stop taking their Metformin. FDA assured that the organization will continue to monitor NDMA in Metformin, along with other drugs products, and will provide timely updates of new developments, including product recalls. Also, the agency is planning to post the methods used in laboratory testing of Metformin in the near future. For this purpose the FDA is also collaborating with international regulators to share testing results for Metformin, along with testing results for other drugs.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter
