
7. Device & Drug update
First Non-Surgical Weight Loss Procedure for Mild-to-Moderate Obesity
![]()
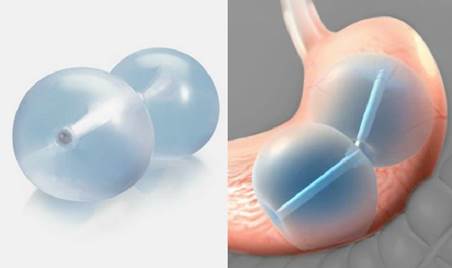 The FDA has approved the first non-surgical weight loss device- ReShape Integrated Dual Balloon System for people with mild-to-moderate obesity. It is intended for adults with a BMI of 30-40 kg/m2 who have one or more obesity related health conditions such as hypertension, dyslipidemia or diabetes and have had unsuccessful weight loss with diet and exercise alone.
The FDA has approved the first non-surgical weight loss device- ReShape Integrated Dual Balloon System for people with mild-to-moderate obesity. It is intended for adults with a BMI of 30-40 kg/m2 who have one or more obesity related health conditions such as hypertension, dyslipidemia or diabetes and have had unsuccessful weight loss with diet and exercise alone.
The device should not be used in patients who are pregnant or using aspirin daily, who have previous GI or bariatric surgery or in patients who have been diagnosed with inflammatory bowel disease, large hiatal hernia, or symptoms of delayed gastric emptying or active H. Pylori infection.
The ReShape Integrated Dual Balloon System is placed endoscopically during a 20 minute outpatient procedure. Patients first receive conscious sedation (light anesthesia) to relax them and make them comfortable, then the doctor places an endoscope into the mouth and advances it through the esophagus and into the stomach. The doctor inspects the stomach to make sure there are no conditions that would prevent placement of the balloon. The uninflated ReShape Dual Balloon device is inserted into the mouth and advanced through the esophagus and into the stomach. Each balloon is filled with a saline solution that contains a small amount of blue dye. Once both balloons are filled and the endoscope is removed, the patient is moved to the recovery area and is usually able to go home within an hour.
![]() The side effects included discomfort linked to the sedation needed during the insertion procedure, and "rare cases" of severe allergic reaction, heart attack, esophageal tear, infection and breathing difficulties. Patients may experience vomiting, nausea, abdominal pain, gastric ulcers and feelings of indigestion,
Obese patients who had the ReShape Balloon procedure lost 6.4 kg compared to the control group, which lost 3.2 kg on diet and exercise alone. Six months after the balloon was removed, patients treated with ReShape Balloon kept off an average of 4.4 kg of the 6.4 kg they lost.
The side effects included discomfort linked to the sedation needed during the insertion procedure, and "rare cases" of severe allergic reaction, heart attack, esophageal tear, infection and breathing difficulties. Patients may experience vomiting, nausea, abdominal pain, gastric ulcers and feelings of indigestion,
Obese patients who had the ReShape Balloon procedure lost 6.4 kg compared to the control group, which lost 3.2 kg on diet and exercise alone. Six months after the balloon was removed, patients treated with ReShape Balloon kept off an average of 4.4 kg of the 6.4 kg they lost.
 Lixilan found effective in clinical trial
Lixilan found effective in clinical trial
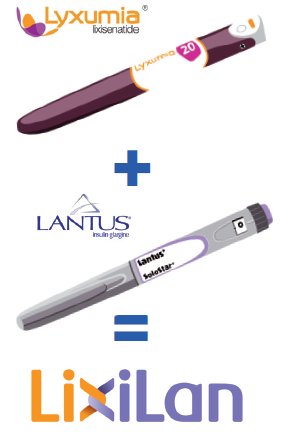
![]() In a clinical trial evaluating the efficacy and safety of LixiLan compared to treatment with either Lantus or Lyxumia alone, LixiLan was found to be effective in reducing average blood glucose levels when compared to Lantus and Lyxumia. LixiLan is a fixed-ratio single daily injection combination of Lyxumia (lixisenatide) and Lantus (insulin glargine) for patients with type 2 diabetes.
In a clinical trial evaluating the efficacy and safety of LixiLan compared to treatment with either Lantus or Lyxumia alone, LixiLan was found to be effective in reducing average blood glucose levels when compared to Lantus and Lyxumia. LixiLan is a fixed-ratio single daily injection combination of Lyxumia (lixisenatide) and Lantus (insulin glargine) for patients with type 2 diabetes.
Semaglutide completes phase 3 trial
![]() Semaglutide is a once weekly GLP-1 agonist. In SUSTAIN-3 trial which evaluated the efficacy and safety of 0.5mg and 1.0mg semaglutide as a monotherapy compared with placebo for a treatment period of 30 weeks, Semaglutide was found to be efficacious when compared to placebo.
Semaglutide is a once weekly GLP-1 agonist. In SUSTAIN-3 trial which evaluated the efficacy and safety of 0.5mg and 1.0mg semaglutide as a monotherapy compared with placebo for a treatment period of 30 weeks, Semaglutide was found to be efficacious when compared to placebo.
![]() The participants were randomized to receive 0.5mg, 10mg Semaglutide or placebo. Results showed that 74% of patients in the 0.5 group and 73% patients in the 1.0mg achieved A1c below 7% compared to only 25% of the placebo group. A reduction of 1.5% and 1.6% in A1c was observed in the 0.5mg and 1.0mg, respectively. Patients treated with semaglutide in either doses of 0.5 mg or 1.0 mg experienced a superior weight loss of 3.8 kg and 4.6 kg, respectively, compared with a weight loss of 1.0 kg for people treated with placebo.
The participants were randomized to receive 0.5mg, 10mg Semaglutide or placebo. Results showed that 74% of patients in the 0.5 group and 73% patients in the 1.0mg achieved A1c below 7% compared to only 25% of the placebo group. A reduction of 1.5% and 1.6% in A1c was observed in the 0.5mg and 1.0mg, respectively. Patients treated with semaglutide in either doses of 0.5 mg or 1.0 mg experienced a superior weight loss of 3.8 kg and 4.6 kg, respectively, compared with a weight loss of 1.0 kg for people treated with placebo.
FDA approves PCSK9 inhibitor Evolocumab (Repatha)
![]() One more lipid-lowering drug, the PCSK9 inhibitor evolocumab (Repatha) has been granted approval by the FDA.Evolocumab is approved for use in patients who fail to achieve LDL cholesterol lowering through diet and maximally-tolerated statin therapy in adults who require additional LDL cholesterol lowering, including patients with heterozygous or homozygous familial hypercholesterolemia.Alirocumab (Praluent), another PCSK9 inhibitor was approved last month.
One more lipid-lowering drug, the PCSK9 inhibitor evolocumab (Repatha) has been granted approval by the FDA.Evolocumab is approved for use in patients who fail to achieve LDL cholesterol lowering through diet and maximally-tolerated statin therapy in adults who require additional LDL cholesterol lowering, including patients with heterozygous or homozygous familial hypercholesterolemia.Alirocumab (Praluent), another PCSK9 inhibitor was approved last month.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter
