 This original research was presented at the American Diabetes Association 72nd Scientific Sessions at Philadelphia in June 2012 from Jothydev’s Diabetes Research Center. This original research was presented at the American Diabetes Association 72nd Scientific Sessions at Philadelphia in June 2012 from Jothydev’s Diabetes Research Center.
 We evaluated efficacy and safety of liraglutide in Indian patients with type 2 diabetes mellitus (T2DM) at a Diabetes Research Center in South India in real-life situation. We retrospectively analyzed case records of 195, T2DM patients (male: 108; female: 87) receiving liraglutide therapy for at least 6 months. Patients were followed up weekly via Diabetes Tele Management System (DTMS®) by a multidisciplinary team of doctors, dietitians, nurses etc. We evaluated efficacy and safety of liraglutide in Indian patients with type 2 diabetes mellitus (T2DM) at a Diabetes Research Center in South India in real-life situation. We retrospectively analyzed case records of 195, T2DM patients (male: 108; female: 87) receiving liraglutide therapy for at least 6 months. Patients were followed up weekly via Diabetes Tele Management System (DTMS®) by a multidisciplinary team of doctors, dietitians, nurses etc.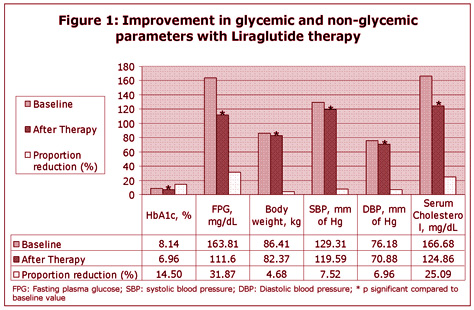 The mean age, weight and mean duration of T2DM were 45.87 years, 86.41 kg and 6.48 years respectively. Liraglutide was administered as 1.8mg OD in addition to prior oral antidiabetic drugs/insulin or both along with other medications for coexisting disorders. The mean age, weight and mean duration of T2DM were 45.87 years, 86.41 kg and 6.48 years respectively. Liraglutide was administered as 1.8mg OD in addition to prior oral antidiabetic drugs/insulin or both along with other medications for coexisting disorders.
With 6 months of liraglutide therapy, significant reduction in glycemic and non-glycemic parameters were noted (Figure 1). Total 49.23% and 37.95% patients achieved HbA1c reduction <7% and <6.5% respectively. Weight loss ≥ 10 kg was noted in 7.18% patients.
 Twenty two (11.28%) patients reported adverse events (AE), most common (>1%) being vomiting (3.08%); tiredness (2.05%); loose motion (1.54%); nausea, flatulence, headache, giddiness (all 1.03%). All were mild to moderate & none were hospitalized. 3.08% patients withdrew due to AE. Liraglutide is relatively new addition to existing antidiabetic therapy with a multitude of benefits. In selective Indian T2DM patients it provides significant improvement in glycemic control, reduction in body weight, blood pressure, cholesterol and is well tolerated. Twenty two (11.28%) patients reported adverse events (AE), most common (>1%) being vomiting (3.08%); tiredness (2.05%); loose motion (1.54%); nausea, flatulence, headache, giddiness (all 1.03%). All were mild to moderate & none were hospitalized. 3.08% patients withdrew due to AE. Liraglutide is relatively new addition to existing antidiabetic therapy with a multitude of benefits. In selective Indian T2DM patients it provides significant improvement in glycemic control, reduction in body weight, blood pressure, cholesterol and is well tolerated.
|
