
 You may recall the approval and the eventual ban of the first inhaled insulin from Pfizer named Exubera in 2007.
The U.S FDA has approved the inhaled human insulin product Afrezza to improve glycemic control in adults with type 1 or type 2 diabetes. The FDA said the device offers a new treatment option for patients with type 1 diabetes. The approval broadens the options available for delivering mealtime insulin for diabetes patients.
The drug will carry a boxed warning that acute bronchospasm has been seen in patients with asthma and chronic obstructive pulmonary disease and that it should not be used in patients with those conditions.
You may recall the approval and the eventual ban of the first inhaled insulin from Pfizer named Exubera in 2007.
The U.S FDA has approved the inhaled human insulin product Afrezza to improve glycemic control in adults with type 1 or type 2 diabetes. The FDA said the device offers a new treatment option for patients with type 1 diabetes. The approval broadens the options available for delivering mealtime insulin for diabetes patients.
The drug will carry a boxed warning that acute bronchospasm has been seen in patients with asthma and chronic obstructive pulmonary disease and that it should not be used in patients with those conditions.
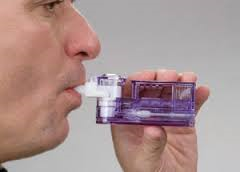
Afrezza is a rapid-acting inhaled insulin to be administered prior to meals or within 20 minutes of starting a meal. It is not a substitute for long-acting insulin and must be used in combination with long-acting insulin in patients with type 1 diabetes. It is not recommended for the treatment of diabetic ketoacidosis or in patients who smoke or who have chronic lung disease.
Efficacy and safety data came from studies involving a total of 3,017 patients, including 1,026 with type 1 and 1,991 with type 2 diabetes. At 24 weeks, Afrezza reduced HbA1clevels by the prespecified end point of 0.4% points in both groups. Hemoglobin A1c reduction was inferior to that of insulin aspart among type 1 patients but significantly superior to placebo among type 2 patients who were also taking oral glucose-lowering medications.
The most common adverse reactions associated with Afrezza in clinical trials were hypoglycemia, cough, and throat pain or irritation.
Afrezza will come in a powder form with a small inhaler which is pocket sized and easy to train and use. Different doses come in multiple cartridges which each contain a single dose that is disposable after use.
Afrezza may also not be available for a while as MannKind is seeking a partner with a pharmaceutical company for distribution.
