7. Drug Updates |
Data of Sotagliflozin presented at ADA
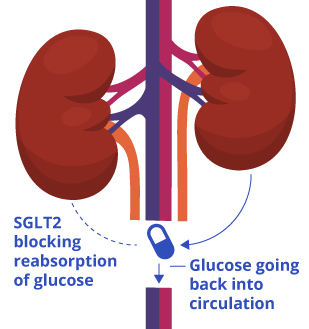
![]() Lexicon Pharmaceuticals announced data from its two pivotal sotagliflozin studies, inTandem1 and inTandem2, in type 1 diabetes at the 77th American Diabetes Association (ADA) Scientific Sessions in San Diego, CA. Sotagliflozin is a first-in-class, oral dual inhibitor of SGLT1 and SGLT2. SGLT1 is responsible for glucose absorption in the gastrointestinal tract, and SGLT2 is responsible for glucose reabsorption by the kidney.
Lexicon Pharmaceuticals announced data from its two pivotal sotagliflozin studies, inTandem1 and inTandem2, in type 1 diabetes at the 77th American Diabetes Association (ADA) Scientific Sessions in San Diego, CA. Sotagliflozin is a first-in-class, oral dual inhibitor of SGLT1 and SGLT2. SGLT1 is responsible for glucose absorption in the gastrointestinal tract, and SGLT2 is responsible for glucose reabsorption by the kidney.
![]() Collectively, 24-week data from the two pivotal inTandem1 and inTandem2 studies for sotagliflozin in patients with type 1 diabetes demonstrated the following:
Collectively, 24-week data from the two pivotal inTandem1 and inTandem2 studies for sotagliflozin in patients with type 1 diabetes demonstrated the following:
![]()
- Sotagliflozin 200 mg and 400 mg on top of optimized insulin significantly reduced A1C compared to placebo (p<0.001 in both studies).
- Sotagliflozin was generally well tolerated. In both studies, there was a low rate of severe hypoglycemia, which occurred less frequently on sotagliflozin than placebo in three of four arms across the two studies. In both studies, there was a low diabetic ketoacidosis (DKA) rate (0.4% to 3.1% over 24 weeks) that was higher for patients on insulin pump versus multiple dose injections (MDI).
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter

