
7. Drug & Device Updates |
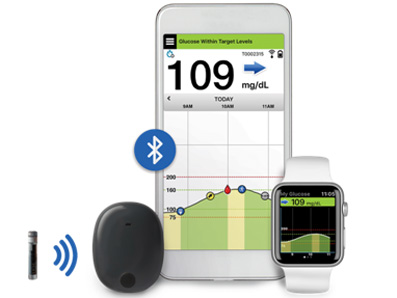
![]() The U.S. Food and Drug Administration has approved the Eversense® Continuous Glucose Monitoring (CGM) system for use in people 18 years of age and older with diabetes. This is the first and only CGM system to feature an implantable glucose sensor and provides long-term continuous monitoring for up to 90 days.
The U.S. Food and Drug Administration has approved the Eversense® Continuous Glucose Monitoring (CGM) system for use in people 18 years of age and older with diabetes. This is the first and only CGM system to feature an implantable glucose sensor and provides long-term continuous monitoring for up to 90 days.
![]() The system includes a pill-sized sensor implanted in the upper arm (by a physician in a short office procedure) for 90 days, an on-body transmitter, and a mobile app that displays glucose data and issues alerts to high and low blood glucose values. Eversense’s 90-day wear thus eliminates the need for frequent sensor insertions required by other CGMs, which currently last only for 7-14 days. This is also the first CGM to issue on-body vibration alerts and thus notifies the user in the event of highs and lows – a particularly useful feature for individuals who are visually impaired or have trouble hearing.
The system includes a pill-sized sensor implanted in the upper arm (by a physician in a short office procedure) for 90 days, an on-body transmitter, and a mobile app that displays glucose data and issues alerts to high and low blood glucose values. Eversense’s 90-day wear thus eliminates the need for frequent sensor insertions required by other CGMs, which currently last only for 7-14 days. This is also the first CGM to issue on-body vibration alerts and thus notifies the user in the event of highs and lows – a particularly useful feature for individuals who are visually impaired or have trouble hearing.
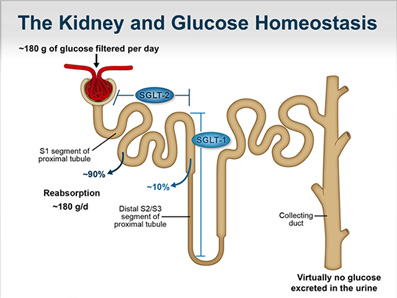
![]() Latest data were presented at the American Diabetes Association (ADA) 2018 Scientific Sessions for the studies inTandem1 (a study of the investigational dual SGLT1/SGLT2 inhibitor sotagliflozin as an adjunct to optimized insulin therapy in T1D adults) and DEPICT-2 (a trial of the SGLT2 inhibitor dapagliflozin along with insulin in T1D adults). In both the trials, the drugs significantly improved HbA1c without increasing hypoglycemia and reduced the glycemic variability and weight. However, in both trials, the agents caused a small but significantly elevated risk for Diabetic Ketoacidosis (DKA).
Latest data were presented at the American Diabetes Association (ADA) 2018 Scientific Sessions for the studies inTandem1 (a study of the investigational dual SGLT1/SGLT2 inhibitor sotagliflozin as an adjunct to optimized insulin therapy in T1D adults) and DEPICT-2 (a trial of the SGLT2 inhibitor dapagliflozin along with insulin in T1D adults). In both the trials, the drugs significantly improved HbA1c without increasing hypoglycemia and reduced the glycemic variability and weight. However, in both trials, the agents caused a small but significantly elevated risk for Diabetic Ketoacidosis (DKA).
![]() Cases of SGLT2 inhibitor-associated DKA have also been previously reported in patients with T2D, and among patients with T1D given the drugs off-label (this class of drugs is not currently approved for treating T1D), and the US FDA had issued a warning about the risk for all drugs in the class. However, the investigators claim that the overall benefits of the drug class outweigh the risk for DKA and other main adverse effects, genital infections and diarrhea (with sotagliflozin). They also opine that the DKA risk can be alleviated with adequate provider and patient training.
Cases of SGLT2 inhibitor-associated DKA have also been previously reported in patients with T2D, and among patients with T1D given the drugs off-label (this class of drugs is not currently approved for treating T1D), and the US FDA had issued a warning about the risk for all drugs in the class. However, the investigators claim that the overall benefits of the drug class outweigh the risk for DKA and other main adverse effects, genital infections and diarrhea (with sotagliflozin). They also opine that the DKA risk can be alleviated with adequate provider and patient training.
For enquiries info@jothydev.net.
Please visit: jothydev.net | research.jothydev.com | diabscreenkerala.net | jothydev.com/newsletter
